Gandhi Medicos: Exporter & Wholesaler For Pharmaceuticals
The leading exporters and wholesaler of speciality medicines.
For Quality and Cost Effective Solution.
Exporter & Wholesaler in Pharmaceutical
The leading exporters and wholesaler of speciality medicines.
For Quality and Cost Effective Solution.

Gandhi Medicos
SPECIALISING IN LIFE-SAVING MEDICINES.
Gandhi Medicos -India, founded in 2006 in the capital of the India – New Delhi, is a one-point solution for all types of Cancer, Hepatitis-C, Hepatitis-B & HIV Medicines. We also deal in other medicines like Kidney & Liver Transplant, Injection & Life Saving Drugs, Nephrology Drugs, and other Pharmaceutical Capsules & Tablets from various companies. Gandhi Medicos also offers Health Monitors and Critical Care products. Gandhi Medicos sells products in Indian & international market.


THE MARKET LEADERS
At Gandhi Medicos, We deal with the Top Pharma Brands in the World.
Gandhi Medicos is the leading pharmaceutical Exporter, Supplier & Wholesale online Pharmacy of Generic Medicine.
Exporter, Supplier & Wholesale of Top Brands




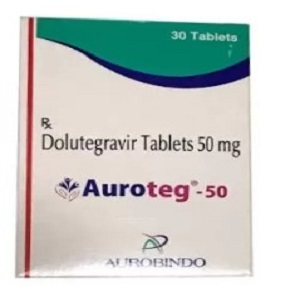
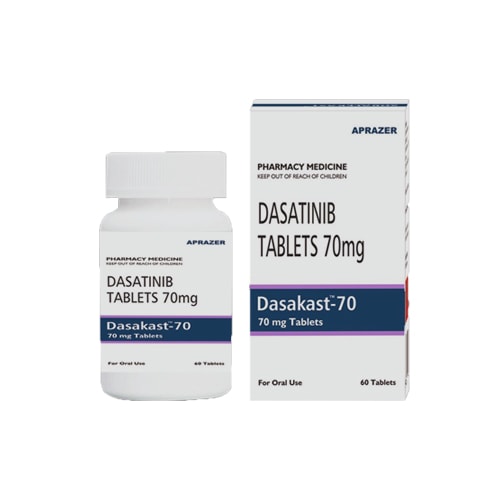
Top Inquired Products
MEDICINE RANGE
TOP CATEGORIES

LATEST PRODUCTS

21900
Happy Customers
326
Products
311
Brands
190
Countries
READ HEALTHCARE NEWS
THE LATEST BLOGS BY GANDHI MEDICOS
Latest healthcare news and tips to stay fit .





 +91-9811604444/ 9811604424/ 9999064250
+91-9811604444/ 9811604424/ 9999064250  8(800)100-47-90
8(800)100-47-90